Recently in science we have been learning about energy changes in reactions and addition polymerisation.
Energy changes in reactions
When a chemical reaction occurs, energy is transferred from or to the surroundings. After this has happened, there is often a temperature change. There are two types of reactions, they are: endothermic reactions and exothermic reactions.
Exothermic reactions are reactions that transfer energy to the surroundings. Exothermic reactions result in a temperature increase. Examples of exothermic reactions are:
• Neutralisation reactions between acids and alkalis.
• The reaction between water and calcium oxide.
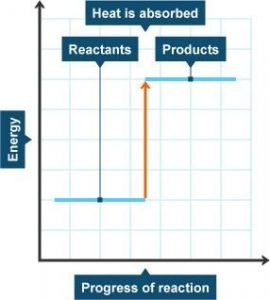
Energy is either taken in or given out during a chemical reaction. During an exothermic reaction, energy is given out. When the energy is given out it means the products have less energy than the reactants, this can be shown using an energy-level diagram. During an endothermic reaction, energy is taken in. If it’s an endothermic reaction the products will be higher than the reactants. Below is an energy level diagram showing endothermic reactions.

Addition polymerisation
Polymers are large chain of monomers joined together. Monomers are simple molecules that make up Polymers. Alkenes (methane, ethene, propene and butene) are able to act as monomers because they contain a double bond. They join end-to-end during the reaction called polymerisation. They polymers they form are called ‘addition polymers’.
The equation for addition polymers = a lot of monomers → a polymer molecule
Example 1 – with detail
Let’s start with propene and polypropene. The reaction which will take place is:
• Propene → poly(propene)
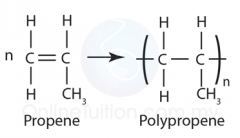
Shown below is the displayed formula for propene. In the previous blogs you should of have read how to draw and work out the displayed formula for alkenes. As you can see propene has 3 carbon atoms and 6 hydrogen atoms. The first carbon atom has 2 hydrogen atoms and is connected to a double bond which leads on to the next carbon atom with 1 hydrogen atom, then from there the final carbon atom which contains the final 3 hydrogen atoms.
Also below is the displayed formula for propene → poly(propene). As you can by the diagram to make the reaction propene → poly(propene), you have to take the propene molecule and bunch 1 carbon atom and 3 hydrogen atoms together to make CH3. After you have done that, to make the poly(propene), you take the exact same displayed formula as propene but this time you have to remove the double bond and put brackets around the formula. Once you have put the brackets in you then put a little n on the outside of it.

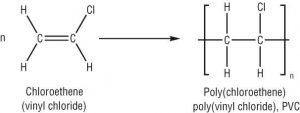
Shown below is the addition polymerisation reaction of polychloroethene
Uses of polyethene include:
• Material of plastic bags
• Material of dustbins
• Material of washing up bowls
• Clingfilm
• A drink bottles
• Strong material
• Easy to shape (malleable)
• Transparent
Uses of polypropene include:
• Material of certain floor tiles
• Milk bottles
• Making cable ties
• Very strong material
• Stronger than Polyethene
• More rigid than Polyethene
Questions based on these topics.
1. If you have a negative value what type of reaction do you get?
2. If you have a positive value what type of reaction do you get?
3. What is polymerisation?
4. What makes up polymers?
5. What is a monomer?
6. If you have Polybutene, what does polymerisation turn it into?
7. If you have chloroethene, what does polymerisation turn it into?
8. What does exothermic mean?
9. What does endothermic mean?
10. What term is used when describing the starting chemicals in a reaction?
Answers
1. An exothermic reaction.
2. An endothermic reaction.
3. Polymerisation is the reaction that makes polymers.
4. Long chains of monomers make up Polymers.
5. A monomer is a single molecule.
6. Butene.
7. Polychloroethene.
8. Exothermic is an increase in temperature.
9. Endothermic is a decrease in temperature.
10. Reactant.